I run an MRI machine!
As an organic chemist, I get to work with all kinds of cool equipment. But one of my favourites is, without doubt, the nuclear magnetic resonance machine.
What's that?
If you've heard of magnetic resonance imaging, then you know at least a bit about nuclear magnetic resonance (NMR). It's essentially the same technology. When you have an MRI performed, they're essentially putting you into a large NMR machine, and performing a proton NMR.
How does this work?
An NMR machine works by putting the sample into an intense magnetic field. Most compounds contain hydrogen, and it can have one of two spins, either aligned with or against the field. Most of the time, all the nuclei are aligned with the field. Now we add a little bit of energy in the form of radio waves, and some of the nuclei are forced to align against the field (think about them like little bar magnets). The nuclei aren't really too happy about this though, and jump back to the ground state. In the process, they release a bit of energy. The useful data is obtained by measuring the amount of energy released as the protons go back to the ground state, and how long they took to do so.
To keep the magnet working, it's kept at temperatures of around -269 deg. C, in liquid helium. The magnet is so strong it'll immediately erase a credit card, and no steel tools can be brought remotely close it it.
How do you run an NMR?
Disclaimer: Never run an NMR if you wear a pacemaker or prosthetic limbs. The intense magnetic fields can cause these devices to malfunction.
Firstly, I dissolve the sample in a "deuterated" solvent. An example of that would be heavy water, D2O, or deuterated chloroform, CDCl3. Ordinary solvents containing hydrogen can't be used, as they would show a large peak on the final spectrum, that risks hiding sample peaks. Then I place the sample in an NMR tube, which is about 20 cm long and 5mm in diameter. Then I place the tube in a "spinner" (essentially a holder; the NMR tubes don't spin in a modern machine) and clean the tube with iso-propanol to remove fingerprints. Then, I place the sample into one of the slots in the NMR machine, and do the following:
*Insert: This places the sample into the magnet.
*Lock: This tells the spectrometer which solvent I'm using. The most common one is CDCl3.
*Shim: This aligns the sample so that it's perfectly aligned in the magnet
*New experiment: sets the parameters for a new experiment, such as the nucleus I'm scanning (I can do fluorine, carbon, aluminum, and a host of other elements if I so desire...), number of scans, relaxation delay, and more.
*Tune probe: Tunes the probe to "listen" to the nuclei I'm interested in. It's like tuning a radio to the correct station. In fact, some experiments used to be affected by a nearby radio station (it left a bit of noise on the spectrum).
*Start: runs the experiment.
*Eject: returns the NMR tube.
The next step is to process the spectrum. I won't go into details of that here...
Enough talk! Show me some spectra!
You ask, we deliver! These are spectra I took myself.
Methyl ethyl ketone, a common additive in adhesives: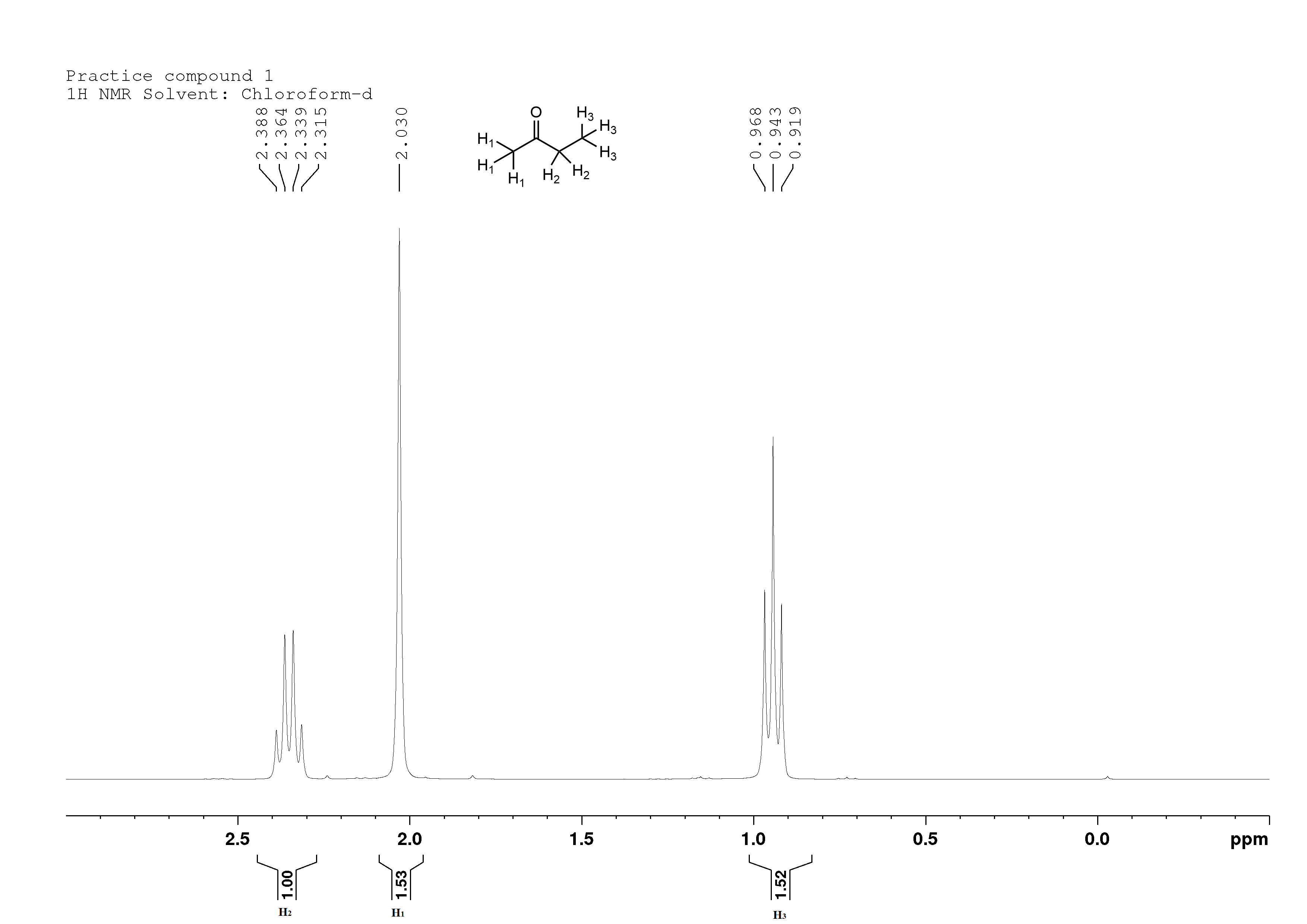
Butyl acetate, contributes to the smell of apples: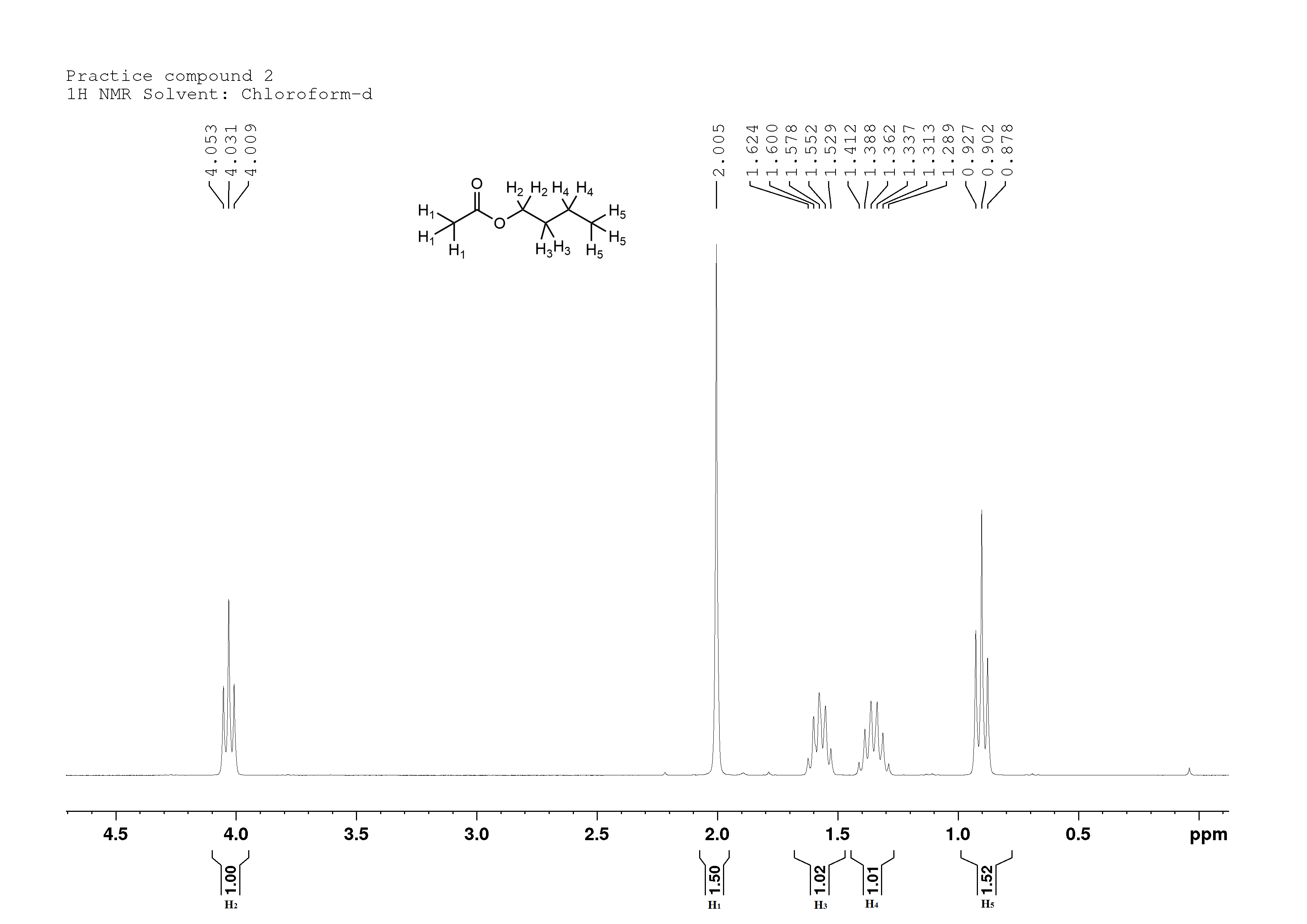
Cinnamyl alcohol, an agent used in perfume: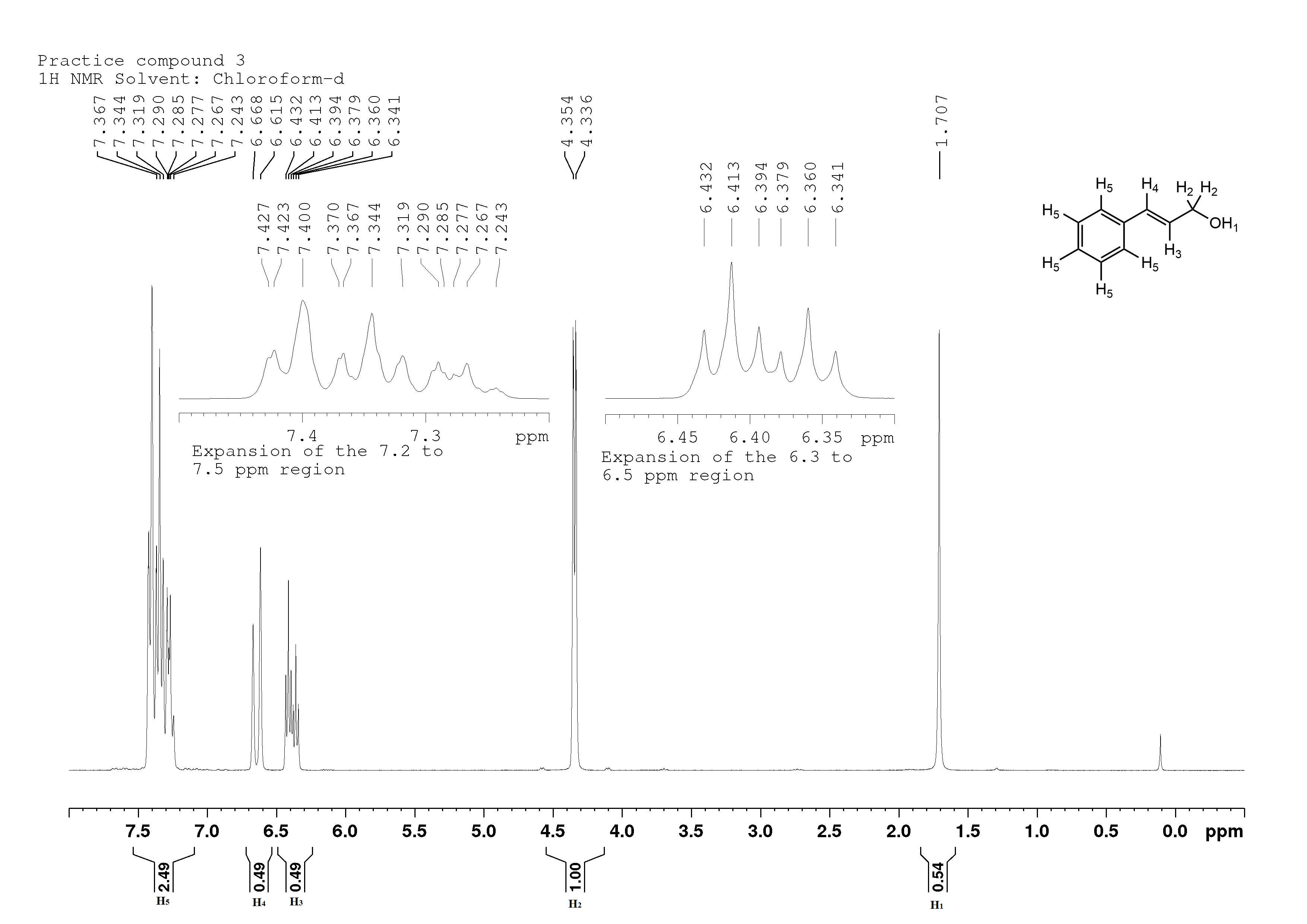
What do all those peaks mean?
Well, since I know a lot of you enjoy patterns, you might find the spectra enjoyable for that reason alone. However, here's how you would interpret spectra...
2-butanone
You may have heard of methyl ethyl ketone before, and this is what they were talking about. It's a common solvent found in adhesives, many of which dry as the solvent evaporates.
Looking at the spectrum we see that two peaks have an integration value of around 1.5. That doesn't make sense (you can't have half a hydrogen atom in a compound), so we double the area of all peaks.
H1 is a singlet at 2.0 ppm. That's because the carbon it's next to (the ketone) doesn't have any protons on it, and the carbonyl group is electronegative, so it deshields the protons on neighbouring carbon atoms (shifts the peaks towards the left of the spectrum). It has an integration value of 3, because there are three protons on that carbon.
H2 is a quartet at 2.35 ppm. Like H1 it is next to the ketone, so it's quite deshielded. It is even more deshielded than H1, though, because the carbon atom is on only has two hydrogens attached to it (making it a secondary carbon). Hydrogens on secondary carbons are always more deshielded than those on primary carbons (those with three hydrogens attached to them). It is split into a quartet by the three H3's, and has an integration value of 2, because there are two hydrogens on that carbon.
H3 is a triplet at 0.9 ppm. It's located farther away from the ketone, so it's not as deshielded. It is split into a triplet by the two H2's, and has an integration value of 3, due to the three protons.
Butyl acetate
This compound occurs naturally in apples, and contributes to their taste and odour.
Again, I see two peaks with an integration value of 1.5, so I will double the area under all the peaks.
H1 is a singlet at 2.0 ppm, for the same reasons as in the 2-butanone. Integration value is 3, also for the same reasons.
H2 is a triplet at 4.0ppm. It's very close to the oxygen of the ester, so it is very deshielded. It is split into a triplet by the two H3's and its integration value is 2 for two protons.
H3 is a quintet at 1.6 ppm. It's still kind of close to the oxygen of the ester, so it's still deshielded, but not as much. It is split into a quintet by the two H2's and the two H4's. Integration value is 2, for two protons.
H4 is a sextet at 1.35 ppm. It's farther still from the oxygen, so it's even less deshielded than H3. The sextet arises from being split by the two H3's and the three H5's (I hope you're starting to see the n+1 splitting rule by this point, where the number of times a proton is split is equal to the number of protons on neighbouring carbons plus one). Interation value is 2 for two protons.
H5 is a triplet at 0.9 ppm. It really isn't very deshielded at all, and is split into a triplet by the two H4's. Integration value 3, for three protons.
Cinnamyl alcohol
This compound is made by the sodium borohydride reduction of cinnamaldehyde which (surprise!) is what gives cinnamon its flavour and fragrance.
Again, I see peaks with an integration value of 0.5 and 2.5, so I'll double the values of the peaks once more.
H1 is a singlet at 1.7 ppm (note: OH chemical shift can vary greatly). It is located on the OH functional group, so it isn't split by anything. Integration value is 1, for one proton. If I were to add some D2O (heavy water), this signal would completely disappear as the proton is replaced by deuterium, which doesn't show up in a proton NMR. That's also why I use deuterated chloroform (CDCl3) as my solvent -- otherwise I'd see a massive peak from the H in the CHCl3.
H2 is a doublet at 4.4 ppm, deshielded by the nearby OH, and split by the single H3. Its integration value is 2, so there are two protons there.
H3 is interesting. It might appear to be a sextet, but it's actually a doublet of triplets. Its signal is first split into a doublet by the lone H4, then each of those doublets is split again by the two H2's. Why doesn't the signal split up into a quartet? It's because the H4 is closer to H3 than H2 is to H3. That's because a carbon-carbon double bond, as seen between the carbons that H3 and H4 are attached to, is shorter (and stronger) than a carbon-carbon single bond, as seen between the carbons that H2 and H3 are attached to, so the signal gets split first by the nearer protons. (Actually -- it's not quite that simple. The coupling constant for protons on an alkene is 15 Hz, while it's 7 Hz for protons on a regular alkane. That's the actual reason why you see that doublet of triplets. However, my model is likely more intuitive. Remember, all models are wrong, but some models are useful). Integration value is 1, for a single proton.
H4, at 6.6ppm, is split into a doublet by the single H3. However, the two peaks are father apart than usual, due to the larger coupling constant of an alkene. Integration value is 1, for a single proton.
H5, at 7.3 ppm, is split into a multiplet. There is no electron withdrawing group close enough to the ring (the alkene isn't very electron withdrawing, and the OH is too far away), so it's impossible to distinguish between the five protons on that ring, although it is possible in some cases when an electronegative group is right next to the ring (benzoic acid for example). The integration value is 5, for five protons.
If you have any questions, please ask!
Oh, and I do admit, I did make the title a bit clickbaity...
_________________
~Glflegolas, B.Sc.
The Colourblind Country Chemist & Tropical Tracker
Myers-Briggs personality: The Commander
Asperger's Quiz: 79/111, both neurodiverse and neurotypical traits present. AQ score: 23 Raads-r score: here
If you have read through all of this and haven't fallen asleep yet, you deserve a cookie. Or at least a new NMR tube...
_________________
~Glflegolas, B.Sc.
The Colourblind Country Chemist & Tropical Tracker
Myers-Briggs personality: The Commander
Asperger's Quiz: 79/111, both neurodiverse and neurotypical traits present. AQ score: 23 Raads-r score: here
Part of my job at the university is to maintain and manage a 300 MHz NMR (5 Tesla magnetic field strength). I have to fill it every week with liquid N2 and every three months with liquid He. It is a pretty nice unit with an autosampler system installed, much better than only running one sample at a time by hand. It has internal shimming to prevent most of magnetic fields from being outside of the unit. The protective warning tape on the floor is only one foot away from the main unit. We also have a 400 MHz NMR in the building, but no one tends to use it because of magnetic drift issues (older system).
In grad school, I was spoiled as I had free control on both a 250 MHz and a 300 MHz for chemical analysis of my research compounds. I did get to run a sample on the 600 MHz once, but that is basically overkill unless you are doing large molecule (protein) research. The older NMR machines there had magnetic field lines that pretty much took up the entire room, so you really had to be careful not to walk into there with any type of ferromagnetic materials. We used to purposely "borrow" other peoples ID cards and wave them into the room to deactivate the magnetic stripe on the cards. The next weekend, they could not get into the building because the card reader would not read their card. Ahh, good times...
I did read that there is a high end watch that has been developed that is basically unaffected by NMR and NMRI pulses. It is on my wish list.
Do you run a Bruker AV300 spectrometer? We have an autosampler on the 500 and use the 300's autosampler during evenings and weekends. The 300 MHz spectrometer has field lines that extend 4ft outwards, the 500's lines extend about 3 ft.
The NMR tech guy once decided to see what happens if you bring a watch too close to a spectrometer. First it stopped. As he got closer, it started running backwards. When he left the room, the watch never re-started.
_________________
~Glflegolas, B.Sc.
The Colourblind Country Chemist & Tropical Tracker
Myers-Briggs personality: The Commander
Asperger's Quiz: 79/111, both neurodiverse and neurotypical traits present. AQ score: 23 Raads-r score: here
I will have to look at it when I get back later this week to be sure (on vacation right now), but that sounds about right. We try to have the NMR going as much as possible for the students. I would like to have it running with samples 24/7 if I could. The unit I maintain currently has a 60 sample changer installed (it used to have a 12). The warning tape around the instrument was put down by the Bruker technicians when they installed it a few years ago. I have brought my stainless steel cased Bulova wristwatch within a foot of it by accident during a fill and it did nothing to it. The newer Bruker instruments have good internal shimming to prevent a majority of the excess magnetic flux fields from escaping the unit.
I did get specialized training at Bruker's main headquarters to help maintain the instrument for the university. If you have not been there, I recommend going just to see the toys that they get to play with in designing the instruments. I would love to have the liquid He recycler (only need to fill once a year) I saw there, but it costs more than the NMR I work with! My most common problem that I have had so far (knock on wood, I do not want to jinx myself) has been with an occasional sample sticking in the autosampler unit. That is a relatively easy fix to do.
One thing I've been curious about medical MRI machines. Perhaps this might not be a bad place to ask.
In the mid 1970s, I had open heart surgery to close up an ASD - Atrial Septal Defect. They used stainless steel suture to close it up. (I usually refer to it as baling wire.)
From what I understand, some stainless steel suture is magnetic to a degree. I've been curious how the stainless steel suture would be affected by the magnetic fields of an MRI machine.
It really depends on the precise alloy that was used. Stainless steel is not created equal. Lower grades of stainless steel are typically magnetic to some degree, but higher-grade stainless steel is not very magnetic at all. I assume that a heart suture would be made out of high-grade stainless steel, so it shouldn't be affected too much (i.e. it won't likely cause any injury), but you might feel it getting attracted towards the magnet.
_________________
~Glflegolas, B.Sc.
The Colourblind Country Chemist & Tropical Tracker
Myers-Briggs personality: The Commander
Asperger's Quiz: 79/111, both neurodiverse and neurotypical traits present. AQ score: 23 Raads-r score: here
I have access to that machine, along with an AV500. The 500's on automation all the time, while the 300's on automation at night. During the day, it's reserved via 10 minute sample bookings.
_________________
~Glflegolas, B.Sc.
The Colourblind Country Chemist & Tropical Tracker
Myers-Briggs personality: The Commander
Asperger's Quiz: 79/111, both neurodiverse and neurotypical traits present. AQ score: 23 Raads-r score: here
Do you have an autosampler system on them? We used to have a 12 sample unit on this one but upgraded to a much better one a few years ago. Man, I can remember having to do one sample right after the other by hand less than a decade ago, along with manual tuning of the instrument. I am glad that it is a thing of the past.
Yes, I have a 60 slot autosampler on the 500 and a 16 slot one on the 300.
_________________
~Glflegolas, B.Sc.
The Colourblind Country Chemist & Tropical Tracker
Myers-Briggs personality: The Commander
Asperger's Quiz: 79/111, both neurodiverse and neurotypical traits present. AQ score: 23 Raads-r score: here
Do you have issues with samples sticking in the autosampler when they get ejected?
On the university's 300 MHz, it happens once every two to three hundred samples or so. If there are no other samples being run immediately after it, not a big problem. However, the NMR often gets stacked with 30-40 samples at a time and that can lead to major issues if one gets stuck. The instrument will automatically shut down the automation unit after three aborted runs in a row. There is a way to cancel the aborted ones and resubmit them back into the system without having to delete them after the problem sample has been removed from the instrument.
If you get a sample sticking issue: It can be fixed by removing the plastic cover on the cassette, gently tapping down on the sample to allow it to go back into the NMR, shutting down the automation unit, removing the cassette and then doing a manual eject via the TopSpin program to retrieve the sample. Easy to do, but PITA if it happens over and over again like this one does.
We also had a power lag earlier this summer that took out the UPS battery to the main unit/autosampler. It took two weeks to get the right battery shipped to the department from the factory. During the wait time, we were forced to do a direct link to the wall outlet (not recommended - no surge protection). We just did not have a spare UPS that could withstand the load needed for the equipment, something that we are likely going to be buying soon. The lag did not affect the other UPS to the main computer and server, so at least it did not mess that up.







